MENU
ZA | ZAR
ZA | ZAR
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifugation Consumables
- Accessories
- Tubes
- Plates
- Device Management Software
- Sample and Information Management
No results found
Search Suggestions

Stable vs transient expression: Which to use and when?
Lab Academy
- Cell Biology
- Cell Culture
- CO2 Incubators
- Essay
This article was published first in Inside Cell Culture , the monthly newsletter for cell culture professionals. Find more interesting articles about CO2 incubators/shakers on our page https://www.eppendorf.link/co2-incubators .
Read more
Read less
An introduction to transient and stable expression
Mammalian expression systems are indispensable tools for synthesizing a wide range of biomolecules, including recombinant proteins, antibodies, and vaccines. Culturing mammalian cells offers distinct advantages, as they are capable of post-translational modifications that closely mimic those in humans. This ensures the proper folding and functionality of complex proteins.
There are two main expression strategies for protein production in mammalian cell lines: transient and stable (Figure 1) [1]. Transient expression involves the temporary introduction of foreign genetic material, in the form of either DNA or RNA, into host cells. Unlike stable expression, transient expression does not result in the permanent integration of foreign genetic material into the host genome, allowing for short-term gene expression without altering the genetic makeup of the host cell [2].
Stable expression involves the integration of foreign DNA into the host cell's genome, resulting in a lasting genetic change that is passed on to cell progeny. This type of expression system requires the careful, multi-step process of selecting and culturing cells that have successfully incorporated the desired genetic material. Following the generation of stable cell lines, these genetically modified cells reliably produce proteins or exhibit specific traits dictated by the introduced genetic material.
For transient and stable expression, there are several different transfection methods that can be used, including physical, biological, or chemical strategies. Common transfection methods can be used for both transient and stable expression, including cationic lipid-based transfection, electroporation, and viral vectors [3]. However, specific cationic polymers such as Diethylaminoethyl-dextran (DEAE-dextran) can only be used in transient expression systems, and are most suitable for RNA transfection. Viral vectors including adenoviruses and adeno-associated virus (AAV) vectors are used to transiently express DNA, whereas lentiviruses are primarily used in stable expression [3].
There are two main expression strategies for protein production in mammalian cell lines: transient and stable (Figure 1) [1]. Transient expression involves the temporary introduction of foreign genetic material, in the form of either DNA or RNA, into host cells. Unlike stable expression, transient expression does not result in the permanent integration of foreign genetic material into the host genome, allowing for short-term gene expression without altering the genetic makeup of the host cell [2].
Stable expression involves the integration of foreign DNA into the host cell's genome, resulting in a lasting genetic change that is passed on to cell progeny. This type of expression system requires the careful, multi-step process of selecting and culturing cells that have successfully incorporated the desired genetic material. Following the generation of stable cell lines, these genetically modified cells reliably produce proteins or exhibit specific traits dictated by the introduced genetic material.
For transient and stable expression, there are several different transfection methods that can be used, including physical, biological, or chemical strategies. Common transfection methods can be used for both transient and stable expression, including cationic lipid-based transfection, electroporation, and viral vectors [3]. However, specific cationic polymers such as Diethylaminoethyl-dextran (DEAE-dextran) can only be used in transient expression systems, and are most suitable for RNA transfection. Viral vectors including adenoviruses and adeno-associated virus (AAV) vectors are used to transiently express DNA, whereas lentiviruses are primarily used in stable expression [3].
Read more
Read less
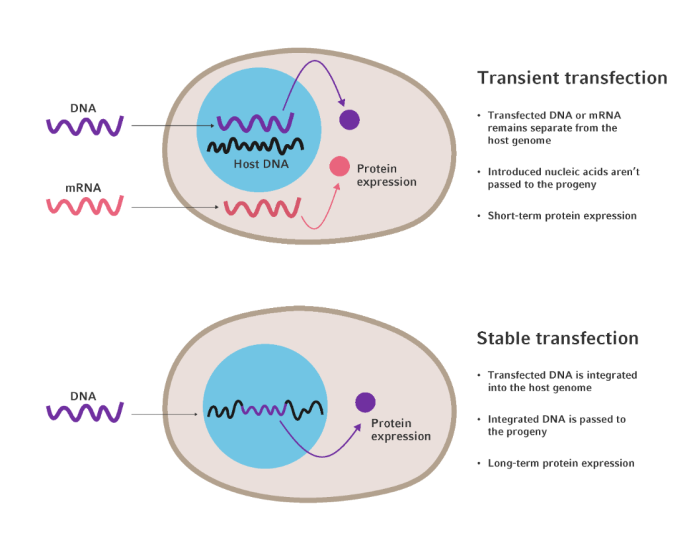
Figure 1. Schematic diagrams representing the mechanisms of transient and stable transfection. In transient transfection, foreign genetic material is delivered to either the cell nucleus or cytosol but isn’t incorporated into the host genome. In stable transfection, foreign DNA is delivered to the cell nucleus and is integrated into the host genome for expression. Adapted from Kim, T. K 2010 [1].
Transient and stable expression systems have their own set of advantages, disadvantages, and useful applications. Understanding the differences between these systems, and knowing when to use each, is essential for optimizing protein expression in your research or production process.
Read more
Read less
Transient Expression
Advantages
- No selective screening or clonal isolation required: Unlike stable expression, transient expression does not require selective screening or isolation steps, saving on time and resources.
- Rapid protein production: One of the major advantages of transient expression is its speed. This type of expression doesn’t require host-genome integration, allowing for the quick production of proteins, particularly when using mRNA transfection methods as transcription is not required. Protein expression has been observed as early as 6 hours post-transfection, and cells can typically be harvested within 24-96 hours of transfection [4].
- Flexibility: Transient expression offers flexibility in experimental design, allowing researchers to easily modify expression constructs or experimental conditions without the need for cell line generation. Additionally, both RNA and DNA transfection methods can be used, providing researchers with a versatile toolkit.
- Lower risk of genomic integration: Since the introduced foreign DNA is not permanently integrated into the host genome, the risk of unintended genetic alterations is minimized in transient expression.
- Cost-effectiveness: Transient expression is generally more cost-effective than stable expression methods, as it requires fewer resources and has shorter experimental timelines, plus high levels of protein expression can often be achieved.
Read more
Read less
Disadvantages
- Transient nature: The transient expression of proteins is short-lived; within a few days, nucleases present in the host cell degrade foreign genetic material. And as the transfected DNA isn’t passed to cell progeny, rounds of cell division dilute the expressed protein, making it more difficult to detect and harvest, and lowering the yield over time.
- Variable expression levels: Protein expression levels can be inconsistent between experiments or batches, due to factors such as varying transfection efficiency or cell viability. To solve this issue and achieve consistent protein yields, several trial rounds for protocol optimization may be required.
- Scale-up challenges: Scaling up transient expression for large-scale protein production can be challenging, making transient expression less cost-efficient at larger scales compared to stable expression systems.
Read more
Read less
Applications
The speed, flexibility, and cost-effectiveness of transient transfection makes it ideal for urgent or time-sensitive experiments. However, the short-lived nature of transient expression makes it most suited to smaller-scale and shorter-term studies.
Transient expression is currently used in a wide range of applications. It serves as a rapid and flexible method for producing proteins, enabling quick functional studies, structural analyses, and biochemical assays. It also facilitates reporter gene assays, allowing real-time monitoring of gene expression dynamics and cell signaling pathways.
In the biopharmaceuticals sector, transient expression plays a crucial role in generating therapeutic proteins, antibodies, and vaccines for preclinical investigations and assay development. Furthermore, this type of expression is useful for elucidating gene functions through knockdown/knockout or overexpression studies, using advanced molecular techniques like RNA interference and CRISPR/Cas9 to study cellular pathways and responses [5].
Additionally, transient expression systems are instrumental in viral vector production, such as lentivirus, adenovirus, or adeno-associated virus (AAV), as well as smaller-scale gene therapy applications. The flexibility and speed of these systems makes them particularly useful for investigating new gene therapies, especially during early stages of product development [6].
Learn more about AAV vector production in this application note
The speed, flexibility, and cost-effectiveness of transient transfection makes it ideal for urgent or time-sensitive experiments. However, the short-lived nature of transient expression makes it most suited to smaller-scale and shorter-term studies.
Transient expression is currently used in a wide range of applications. It serves as a rapid and flexible method for producing proteins, enabling quick functional studies, structural analyses, and biochemical assays. It also facilitates reporter gene assays, allowing real-time monitoring of gene expression dynamics and cell signaling pathways.
In the biopharmaceuticals sector, transient expression plays a crucial role in generating therapeutic proteins, antibodies, and vaccines for preclinical investigations and assay development. Furthermore, this type of expression is useful for elucidating gene functions through knockdown/knockout or overexpression studies, using advanced molecular techniques like RNA interference and CRISPR/Cas9 to study cellular pathways and responses [5].
Additionally, transient expression systems are instrumental in viral vector production, such as lentivirus, adenovirus, or adeno-associated virus (AAV), as well as smaller-scale gene therapy applications. The flexibility and speed of these systems makes them particularly useful for investigating new gene therapies, especially during early stages of product development [6].
Learn more about AAV vector production in this application note
Read more
Read less
Stable expression
Advantages
- Sustained expression: Incorporation of transfected DNA into the host cell genome leads to permanent genetic alterations that are passed on to cell progeny, allowing for stable, sustained expression.
- Reduced variability: Once established, stable cell lines exhibit less variability in protein expression compared to transient expression systems, offering more consistent yields, with less batch-to-batch variability.
- Large-scale production: With sustained long-term protein production, stable cell lines offer greater consistency in yields, and so are more suitable to larger-scale production.
- Enhanced protein folding and stability: Stable expression systems enable more consistent and sustained protein folding with higher stability. Attributed to the prolonged duration of protein production, this allows for proper post-translational modifications and protein maturation, which are essential for achieving biologically active and functional proteins. This supports the production of more structurally complex proteins, such as fusion proteins, and multidomain proteins [7].
Read more
Read less
Disadvantages
Time-consuming: Generating stable cell lines requires more time and effort compared to transient expression. This is due to the multiple steps involved including selection, amplification, and validation of stable cell lines. The requirement of many steps makes stable expression inefficient for small-scale and short-term studies.
Challenging transfection process: The transfection process involved in establishing stable cell lines is typically more challenging compared to transient expression. Achieving successful integration of the expression vector into the host genome is a rare event and may require optimization of transfection conditions, such as cell density, transfection reagents, and selection markers.
Selection pressure: The process of selecting stable cell lines often involves the use of selective pressure, such as antibiotics or specific growth factors. This can introduce additional stress to the cells, potentially affecting their growth and productivity [8].
Integration effects: Integration of exogenous DNA into the host genome may lead to positional effects, potentially reducing gene expression levels and inducing genomic instability. This could lead to chromosomal rearrangements or mutations, which could compromise cell behavior or product quality.
Time-consuming: Generating stable cell lines requires more time and effort compared to transient expression. This is due to the multiple steps involved including selection, amplification, and validation of stable cell lines. The requirement of many steps makes stable expression inefficient for small-scale and short-term studies.
Challenging transfection process: The transfection process involved in establishing stable cell lines is typically more challenging compared to transient expression. Achieving successful integration of the expression vector into the host genome is a rare event and may require optimization of transfection conditions, such as cell density, transfection reagents, and selection markers.
Selection pressure: The process of selecting stable cell lines often involves the use of selective pressure, such as antibiotics or specific growth factors. This can introduce additional stress to the cells, potentially affecting their growth and productivity [8].
Integration effects: Integration of exogenous DNA into the host genome may lead to positional effects, potentially reducing gene expression levels and inducing genomic instability. This could lead to chromosomal rearrangements or mutations, which could compromise cell behavior or product quality.
Read more
Read less
Applications
Stable expression systems offer versatile solutions for a range of scientific endeavors, including large-scale protein production and long-term pharmacology studies. These systems provide a reliable platform for generating significant quantities of therapeutic proteins or industrial enzymes, meeting the demands of biopharmaceutical production and industrial processes [7].
Find out more about large-scale cell culture in bioreactors
By providing consistent levels of protein production over prolonged periods, stable expression facilitates in-depth investigations into genetic regulation mechanisms and enables sustained protein expression for disease modeling and preclinical studies. Despite the complexity and time investment required for setup, stable expression systems deliver reliable protein yields and play a crucial role in advancing our understanding of cellular processes and disease pathways.
Stable expression systems offer versatile solutions for a range of scientific endeavors, including large-scale protein production and long-term pharmacology studies. These systems provide a reliable platform for generating significant quantities of therapeutic proteins or industrial enzymes, meeting the demands of biopharmaceutical production and industrial processes [7].
Find out more about large-scale cell culture in bioreactors
By providing consistent levels of protein production over prolonged periods, stable expression facilitates in-depth investigations into genetic regulation mechanisms and enables sustained protein expression for disease modeling and preclinical studies. Despite the complexity and time investment required for setup, stable expression systems deliver reliable protein yields and play a crucial role in advancing our understanding of cellular processes and disease pathways.
Read more
Read less
Best choice of transient and stable expression lines
Chinese hamster ovary (CHO) and Human Embryonic Kidney 293 (HEK293) cells are the most commonly used mammalian cell types for stable and transient expression. Both can be adapted to suspension growth, and are capable of large-scale production in serum-based and serum-free media.
Despite both types of cells being highly popular, each has its own specific benefits. HEK293 cells are known for their higher transfection efficiency, fast growth, and more complex and human-specific post-translational modifications, while CHO cells provide high genetic stability and scalability. With this in mind, the best choice of cell line is dependent on your specific experimental requirements.
Read our article to help you choose the best cell line for your research
Despite both types of cells being highly popular, each has its own specific benefits. HEK293 cells are known for their higher transfection efficiency, fast growth, and more complex and human-specific post-translational modifications, while CHO cells provide high genetic stability and scalability. With this in mind, the best choice of cell line is dependent on your specific experimental requirements.
Read our article to help you choose the best cell line for your research
Read more
Read less
Transient vs stable expression: Which is best?
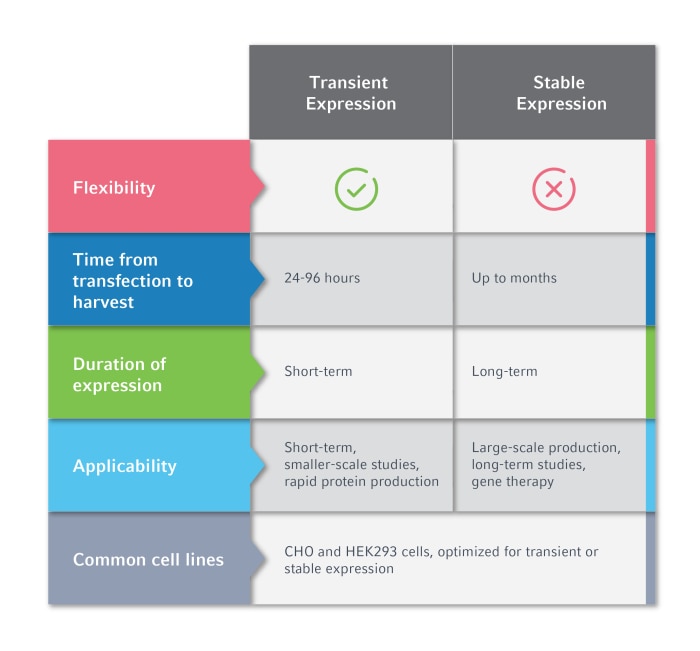
Choosing between stable and transient expression systems depends on several factors, including the desired protein quantity, duration of expression, and scalability requirements.Ultimately, the choice depends on your specific goals, needs, and constraints.
Here's a summary of the main considerations:
Here's a summary of the main considerations:
Read more
Read less
References
1. Kim, T. K., & Eberwine, J. H. (2010). Mammalian cell transfection: the present and the future. Analytical and Bioanalytical Chemistry, 397(8), 3173–3178. https://doi.org/10.1007/s00216-010-3821-6
2. Chong, Z. X., Yeap, S. K., & Ho, W. Y. (2021). Transfection types, methods and strategies: a technical review. PeerJ, 9, e11165. https://doi.org/10.7717/peerj.11165
3. Fus-Kujawa, A., Prus, P., Bajdak-Rusinek, K., Teper, P., Gawron, K., Kowalczuk, A., & Sieron, A. L. (2021). An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro. Frontiers in Bioengineering and Biotechnology, 9, 701031. https://doi.org/10.3389/fbioe.2021.701031
4. T. Juncker, B. Chatton, and M. Donzeau, ‘The Prodigious Potential of mRNA Electrotransfer as a Substitute to Conventional DNA-Based Transient Transfection’, Cells, vol. 12, no. 12, Art. no. 12, Jan. 2023, https://doi.org/10.3390/cells12121591
5. Fajrial, A. K., He, Q. Q., Wirusanti, N. I., Slansky, J. E., & Ding, X. (2020). A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing. Theranostics, 10(12), 5532–5549. https://doi.org/10.7150/thno.43465
6. Wright, J. F. (2009). Transient Transfection Methods for Clinical Adeno-Associated Viral Vector Production. Human Gene Therapy, 20(7), 698–706. https://doi.org/10.1089/hum.2009.064
7. Cell Press: STAR Protocols. (n.d.). Retrieved May, 2024, from https://star-protocols.cell.com/protocols/3094
8. B.-M. Keller, J. Maier, M. Weldle, S. Segan, B. Traenkle, and U. Rothbauer, ‘A Strategy to Optimize the Generation of Stable Chromobody Cell Lines for Visualization and Quantification of Endogenous Proteins in Living Cells’, Antibodies, vol. 8, no. 1, Art. no. 1, Mar. 2019, https://doi.org/10.3390/antib8010010
2. Chong, Z. X., Yeap, S. K., & Ho, W. Y. (2021). Transfection types, methods and strategies: a technical review. PeerJ, 9, e11165. https://doi.org/10.7717/peerj.11165
3. Fus-Kujawa, A., Prus, P., Bajdak-Rusinek, K., Teper, P., Gawron, K., Kowalczuk, A., & Sieron, A. L. (2021). An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro. Frontiers in Bioengineering and Biotechnology, 9, 701031. https://doi.org/10.3389/fbioe.2021.701031
4. T. Juncker, B. Chatton, and M. Donzeau, ‘The Prodigious Potential of mRNA Electrotransfer as a Substitute to Conventional DNA-Based Transient Transfection’, Cells, vol. 12, no. 12, Art. no. 12, Jan. 2023, https://doi.org/10.3390/cells12121591
5. Fajrial, A. K., He, Q. Q., Wirusanti, N. I., Slansky, J. E., & Ding, X. (2020). A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing. Theranostics, 10(12), 5532–5549. https://doi.org/10.7150/thno.43465
6. Wright, J. F. (2009). Transient Transfection Methods for Clinical Adeno-Associated Viral Vector Production. Human Gene Therapy, 20(7), 698–706. https://doi.org/10.1089/hum.2009.064
7. Cell Press: STAR Protocols. (n.d.). Retrieved May, 2024, from https://star-protocols.cell.com/protocols/3094
8. B.-M. Keller, J. Maier, M. Weldle, S. Segan, B. Traenkle, and U. Rothbauer, ‘A Strategy to Optimize the Generation of Stable Chromobody Cell Lines for Visualization and Quantification of Endogenous Proteins in Living Cells’, Antibodies, vol. 8, no. 1, Art. no. 1, Mar. 2019, https://doi.org/10.3390/antib8010010
Read more
Read less

